Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of Adults With Multi-episode Schizophrenia: A Systematic Review and Network Meta-analysis Pregled
Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute
Treatment of Adults With Multi-episode Schizophrenia: A Systematic Review
and Network Meta-analysis
Comparative Efficacy and Tolerability of 32 Oral Antipsychotics for the Acute Treatment of
Adults With Multi-Episode Schizophrenia: A Systematic Review and Network Meta-Analysis
Maximilian Huhn, Adriani Nikolakopoulou, Johannes Schneider-Thoma, Marc Krause, Myrto
Samara, Natalie Peter, Thomas Arndt, Lio Bäckers, Philipp Rothe, Andrea Cipriani, John Davis,
Georgia Salanti, Stefan Leucht
Background: Schizophrenia is one of the most common, burdensome, and costly psychiatric
disorders in adults worldwide. Antipsychotic drugs are its treatment of choice, but there is
controversy about which agent should be used. We aimed to compare and rank antipsychotics
by quantifying information from randomised controlled trials.
Methods: We did a network meta-analysis of placebo-controlled and head-to-head randomised
controlled trials and compared 32 antipsychotics. We searched Embase, MEDLINE, PsycINFO,
PubMed, BIOSIS, Cochrane Central Register of Controlled Trials (CENTRAL), WHO
International Clinical Trials Registry Platform, and
Jan 8, 2019. Two authors independently selected studies and extracted data. We included
randomised controlled trials in adults with acute symptoms of schizophrenia or related
disorders. We excluded studies in patients with treatment resistance, first episode, predominant
negative or depressive symptoms, concomitant medical illnesses, and relapse-prevention
studies. Our primary outcome was change in overall symptoms measured with standardised
rating scales. We also extracted data for eight efficacy and eight safety outcomes. Differences
in the findings of the studies were explored in metaregressions and sensitivity analyses. Effect
size measures were standardised mean differences, mean differences, or risk ratios with 95%
credible intervals (CrIs). Confidence in the evidence was assessed using CINeMA (Confidence
in Network Meta-Analysis). The study protocol is registered with PROSPERO, number
CRD42014014919.
Findings: We identified 54 417 citations and included 402 studies with data for 53 463
participants. Effect size estimates suggested all antipsychotics reduced overall symptoms more
than placebo (although not statistically significant for six drugs), with standardised mean
differences ranging from -0·89 (95% CrI -1·08 to -0·71) for clozapine to -0·03 (-0·59 to 0·52) for
levomepromazine (40 815 participants). Standardised mean differences compared with placebo
for reduction of positive symptoms (31 179 participants) varied from -0·69 (95% CrI -0·86 to
-0·52) for amisulpride to -0·17 (-0·31 to -0·04) for brexpiprazole, for negative symptoms (32 015
participants) from -0·62 (-0·84 to -0·39; clozapine) to -0·10 (-0·45 to 0·25; flupentixol), for
depressive symptoms (19 683 participants) from -0·90 (-1·36 to -0·44; sulpiride) to 0·04 (-0·39
to 0·47; flupentixol). Risk ratios compared with placebo for all-cause discontinuation (42 672
participants) ranged from 0·52 (0·12 to 0·95; clopenthixol) to 1·15 (0·36 to 1·47; pimozide), for
sedation (30 770 participants) from 0·92 (0·17 to 2·03; pimozide) to 10·20 (4·72 to 29·41;
zuclopenthixol), for use of antiparkinson medication (24 911 participants) from 0·46 (0·19 to
0·88; clozapine) to 6·14 (4·81 to 6·55; pimozide). Mean differences compared to placebo for
weight gain (28 317 participants) ranged from -0·16 kg (-0·73 to 0·40; ziprasidone) to 3·21 kg
(2·10 to 4·31; zotepine), for prolactin elevation (21 569 participants) from -77·05 ng/mL (-120·23
to -33·54; clozapine) to 48·51 ng/mL (43·52 to 53·51; paliperidone) and for QTc prolongation
(15 467 participants) from -2·21 ms (-4·54 to 0·15; lurasidone) to 23·90 ms (20·56 to 27·33;
sertindole). Conclusions for the primary outcome did not substantially change after adjusting for
possible effect moderators or in sensitivity analyses (eg, when excluding placebo-controlled
studies). The confidence in evidence was often low or very low.
Interpretation: There are some efficacy differences between antipsychotics, but most of them
are gradual rather than discrete. Differences in side-effects are more marked. These findings
will aid clinicians in balancing risks versus benefits of those drugs available in their countries.
They should consider the importance of each outcome, the patients' medical problems, and
preferences.
Funding: German Ministry of Education and Research and National Institute for Health
Research.
Reference
Huhn, M., Nikolakopoulou, A., Schneider-Thoma, J., Krause, M., Samara, M., Peter, N., Arndt,
T., Bäckers, L., Rothe, P., Cipriani, A., Davis, J., Salanti, G., & Leucht, S. (2019).
The Lancet,
394
(10202), 939-951.
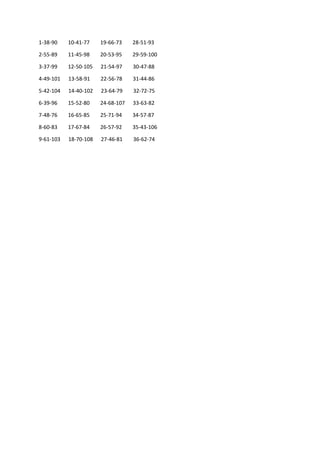
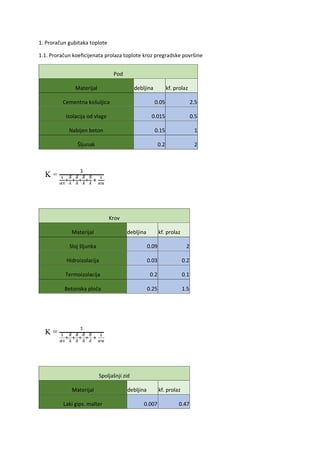
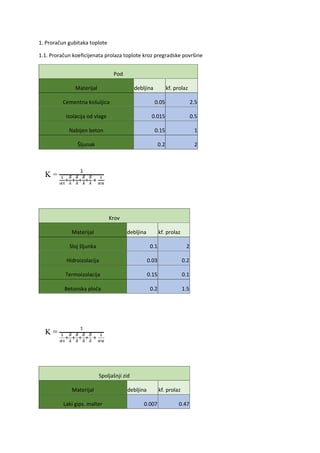
Let’s look at the use of antipsychotics in the treatment of acute schizophrenia. Are some
antipsychotics better than others? Can they be rank-ordered by efficacy and tolerability? This is
similar to the old CATIE trial from the US National Institute of Mental Health. The motive then
was determining whether expensive second-generation antipsychotics were better. Here, the
motivation is that we have many antipsychotics to choose from. Are any of them better than the
others? Thanks to a research team that examined 54,000 publications, selecting 400 for study,
we have a new perspective.
This one is another network meta-analysis similar to Quick Take, Volume 1,
,
which was from the same team. That study was led by Andrea Cipriani, this one by Maximilian
Huhn, each in the Lancet. This technique aggregates data on effect size for medications across
multiple studies, including comparisons against one another, as well as against placebo.
Moreover, the comparisons examined here were not just about efficacy but also positive
symptoms, negative symptoms, social functioning, and the big tolerability problems like weight
gain, akathisia, sedation, among others.
They looked at all of the second-generation antipsychotics licensed in either Europe or the US,
plus 17 first-generation agents, for a total of 32 different medications. The first problem with
interpreting their data is that we surely do not use all 32 of these medications, and some are not
licensed everywhere. We regularly use half of them at most. It is easy to look at the ones that
we regularly use, ignore the rest, and see if we can detect any differences between our routine
medications. Overall, the results somewhat mimic what we clinicians have generally figured out
for the acute phase of schizophrenia, meaning, acute psychosis. The efficacy differences are
quite minimal. Maybe clozapine sticks out a bit, and maybe amisulpride, but US psychiatrists do
not have access to that one. According to this network meta-analysis, amisulpride causes nearly
as much prolactin elevation as risperidone, which led the list for that problem along with its 9-
hydroxy metabolite, paliperidone. Zotepine is high on the efficacy side but worse for weight gain,
worse than clozapine and olanzapine. Notably, quetiapine and risperidone were not too far
behind.
So, US psychiatrists are wondering if they should bemoan their lack of access to sertindole,
which was the lowest on the list for all-cause discontinuation. However, it is not licensed by the
FDA due to sudden deaths in the trials, and in this network meta-analysis, sertindole was
associated with by far the most significant QT prolongation.

Želiš da pročitaš svih 3 strana?
Prijavi se i preuzmi ceo dokument.
Slični dokumenti

Ovaj materijal je namenjen za učenje i pripremu, ne za predaju.